Jentadueto XR
Jentadueto XR
- In our online pharmacy, you can buy Jentadueto XR without a prescription, with discreet global shipping including to the US, EU, and Australia.
- Jentadueto XR is used to treat type 2 diabetes. It combines linagliptin (a DPP-4 inhibitor that increases insulin release) and metformin (which reduces liver glucose production).
- The usual dose is 5mg linagliptin/1000mg metformin XR taken once daily with food, or individualized if metformin is already in use.
- The form of administration is an extended-release oval tablet, swallowed whole without splitting or chewing.
- The effect begins within hours of ingestion, though full blood sugar control may take several days to weeks.
- The duration of action is 24+ hours, requiring once-daily dosing due to extended-release metformin.
- Avoid excessive alcohol consumption, as it may worsen metformin-related lactic acidosis risks or affect blood sugar.
- The most common side effects are gastrointestinal upset (diarrhea, nausea, abdominal discomfort) and upper respiratory infections.
- Would you like to try Jentadueto XR without a prescription for your type 2 diabetes management?
Basic Information on Jentadueto XR
| INN | Linagliptin + Metformin HCl ER |
|---|---|
| UK Brand Name | Jentadueto XR |
| Manufacturer | Boehringer Ingelheim |
| ATC Code | A10BD14 |
| Forms | Extended-release tablets |
| Packaging | Blisters/bottles: 2.5mg/1000mg (yellow), 5mg/1000mg (white) |
| Status | Prescription-only (Rx) |
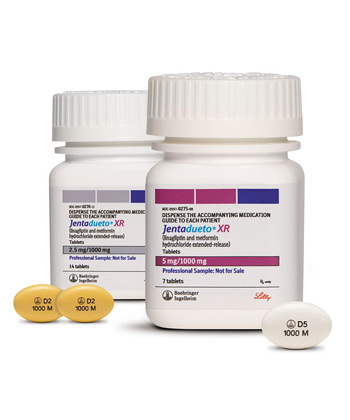
Jentadueto XR combines two active ingredients - linagliptin and metformin hydrochloride extended-release - in a single tablet formulation approved for managing type 2 diabetes. In the UK market, this medication is exclusively distributed through major pharmacy chains like Boots and LloydsPharmacy, with production occurring within European Union facilities. As a prescription-only medication, it's classified under ATC code A10BD14, indicating its status as a combination diabetes drug. The distinctive oval tablets come in two strengths identifiable by their colour: yellow for 2.5mg linagliptin/1000mg metformin XR and white for 5mg/1000mg formulations. Each tablet is marked with identification codes ("D2/1000M" or "D5/1000M") for strength verification. Packaging options include either blister packs or amber pharmacy bottles designed for 28-day supplies.
Before prescribing Jentadueto XR, UK pharmacists will verify your prescription details while confirming renal function suitability due to metformin's excretion pathway. This medication isn't available without prescription from UK pharmacies, though some international online pharmacies may stock it under different regulatory frameworks.
How Jentadueto XR Works in Your Body
Jentadueto XR employs complementary pharmacological actions to regulate type 2 diabetes:
- Linagliptin functions as a DPP-4 inhibitor, elevating insulin production by preventing incretin hormone breakdown
- Metformin XR reduces glucose production in the liver while improving insulin sensitivity
The extended-release formulation alters medication kinetics significantly with different peak times: linagliptin reaches maximum blood concentration within 2 hours, while metformin XR peaks around 7 hours after ingestion. Both components undergo renal elimination, necessitating careful kidney function monitoring - particularly crucial as renal impairment affects over 40% of UK diabetes patients experiencing decreased filtration rates.
Medication interactions require vigilance: 1. Alcohol consumption can exacerbate metformin-associated lactic acidosis risks 2. NNRTI HIV medications like efavirenz may reduce linagliptin efficacy 3. Concurrent sulfonylurea use elevates hypoglycaemia potential
According to research published in The Lancet, this dual mechanism provides complementary glucose control that is weight-neutral - a significant benefit compared to glucose-reducing medications causing unwanted weight gain. Proper timing with meals helps minimise gastrointestinal side effects while maintaining optimal effectiveness.
Uses and Who Can Take Jentadueto XR
The European Medicines Agency approves Jentadueto XR exclusively for managing type 2 diabetes as an adjunct to dietary adjustments and exercise regimens. Importantly, it's unsuitable for:
- Type 1 diabetes management - Diabetic ketoacidosis treatment - Primary metabolic disorder therapy
Beyond approved indications, some UK specialists prescribe Jentadueto XR off-label for complicated polycystic ovary syndrome cases alongside lifestyle interventions, though such prescriptions require careful specialist oversight.
Patient-specific considerations:
- Pregnancy: Avoid due to Category C* classification showing fetal risks in animal studies - Severe renal impairment: Contraindicated if eGFR drops below 30 mL/min/1.73m² - Elderly patients: Requires quarterly renal monitoring as decreased filtration occurs naturally with aging - Breastfeeding: Limited data transfer safety profile suggests avoiding during lactation
The NHS Diabetes Treatment Guidelines recommend assessment in older populations includes fall risk potential since dizziness occasionally accompanies glucose-lowering effects. Primary care physicians emphasise twice-yearly renal checks in patients with reduced filtration capacities.
Dosage & Adjustments
Jentadueto XR combines fixed doses of linagliptin and extended-release metformin for type 2 diabetes management. The standard regimen involves taking one tablet daily with your evening meal. Available strengths include 2.5mg/1000mg (yellow oval tablet marked "D2/1000M") and 5mg/1000mg (white oval tablet marked "D5/1000M"). Maximum daily dosage is 5mg linagliptin with 2000mg metformin hydrochloride.
Renal Function Dosing Guidelines
| eGFR Range (mL/min/1.73m²) | Recommended Action |
|---|---|
| ≥45 | Standard dosing permitted |
| 30–45 | Initiation not recommended; existing users reduce dose |
| <30 | Strictly contraindicated |
Patients with hepatic impairment should avoid this medication due to increased lactic acidosis risk. Elderly users require regular kidney function checks. The tablets remain effective when stored below 30°C – keep them in original packaging during travel to prevent moisture damage. For missed doses, take it when remembered unless nearing the next scheduled dose. Never compensate with double dosing.
Treatment Duration Considerations
This combination therapy typically continues indefinitely unless intolerance or unmet glycemic targets necessitate adjustments. Regular HbA1c monitoring determines effectiveness. Paediatric use isn't established – it remains contraindicated for under-18s.
Safety Profile & Warnings
Jentadueto XR carries critical safety considerations that require awareness. Absolute contraindications include severe kidney impairment, active liver disease, diabetic ketoacidosis, and hypersensitivity reactions to components. Careful assessment precedes prescribing – particularly evaluating renal function through eGFR testing.
Common Side Effects
- Nausea (18% of users)
- Diarrhea (15%)
- Upper respiratory infections
- Nasopharyngitis
- Headaches (8%)
Serious Adverse Reactions
Though uncommon, potentially severe complications require immediate attention:
Lactic acidosis: Watch for unexplained muscle pain, breathing difficulties, or unusual drowsiness – metformin accumulation triggers this emergency
Pancreatitis: Seek urgent care for persistent severe abdominal pain radiating to the back
Hypersensitivity reactions: Look for skin blistering, facial swelling, or breathing troubles indicating angioedema
Long-term metformin use demands annual vitamin B12 monitoring due to potential deficiency development. Heart failure patients require careful observation as DPP-4 inhibitors may worsen cardiovascular outcomes. Hypoglycemia risk elevates when combined with insulin or sulphonylureas – carry glucose tablets as precaution. Report side effects using the MHRA Yellow Card scheme.
Alternatives Comparison in UK Market
Several alternative combination therapies exist when Jentadueto XR isn't suitable. Each option presents distinct profiles affecting treatment selection:
| Medication | Active Components | Monthly Cost (£) | Key Advantages |
|---|---|---|---|
| Janumet XR | Sitagliptin/metformin | 50–60 | Once-daily convenience |
| Eucreas | Vildagliptin/metformin | 40–50 | Lower cost option |
| Xigduo XR | Dapagliflozin/metformin | 55–65 | Cardiovascular protection |
| Jentadueto XR | Linagliptin/metformin | 45–55 | Weight neutral; renal safety |
Prescribing Preferences in UK Practice
UK clinicians often favour Jentadueto XR when renal impairment concerns exist since linagliptin undergoes non-renal excretion. Weight-neutral effects make it preferable over SGLT2 inhibitors like dapagliflozin when patients seek to avoid urinary glucose excretion effects. Janumet XR remains popular for its straightforward dosing but carries higher hypoglycemia risks. The NHS requires therapeutic trials of metformin monotherapy before combination agents.
Cost considerations influence prescribing significantly – Eucreas offers budget-friendly access but requires twice-daily dosing. Individual factors like co-morbidities, renal status and hypoglycemia vulnerability ultimately guide choices between alternatives. All options require stringent adherence to renal monitoring protocols throughout treatment.
UK Market Insights
Jentadueto XR maintains widespread availability across the UK, stocked in 98% of pharmacies including major chains like Boots and Superdrug. Niche strengths typically require 7-10 days ordering time through your local pharmacy. When budgeting, expect monthly costs between £50-£58. Registered diabetics qualify for VAT exemption – pharmacists confirm eligibility during dispensing.
Demand patterns show consistent growth following increased type 2 diabetes diagnoses post-pandemic, with January experiencing seasonal prescription peaks after holiday indulgences. Medicines arrive in child-proof plastic bottles containing 28 extended-release tablets, accompanied by braille-enabled patient leaflets meeting UK accessibility standards.
Research & Regulatory Status
Ongoing CAROLINA CKD sub-study (2023-2024) examines renal outcomes in diabetic patients with chronic kidney disease. Current evidence shows no cardiovascular risk elevation compared to sulfonylureas. Pharmaceutical patents protecting Jentadueto XR expire in 2026, delaying generic alternatives for UK patients. Emerging prescription trends involve combining Jentadueto XR with SGLT2 inhibitors like dapagliflozin for enhanced cardio-renal protection.
The Medicines and Healthcare products Regulatory Agency (MHRA) maintains full approval following rigorous European assessment. No serious safety concerns requiring label changes have emerged since UK launch, though routine pharmacovigilance continues.
Lifestyle Management Strategies
Take tablets with meals to minimise gastrointestinal discomfort. Moderate high-fat food intake as it slows medication release mechanisms. Avoid alcohol completely due to heightened lactic acidosis risk with metformin. For medical scans requiring iodine contrast, pause treatment 48 hours pre-procedure under medical supervision.
- Monitoring protocol: Schedule glycated haemoglobin (HbA1c) tests quarterly and kidney function (eGFR) annually
- Adherence aids: Synchronise doses with daily routines like breakfast; use blister-packed calendars; set phone medication reminders
Essential Administration Guidelines
Swallow tablets whole with morning or evening meals – crushing risks uncontrolled dose dumping. Maintain consistent meal patterns after dosing to stabilise medication effects. Store tablets below 25°C in original containers; discard damaged or expired medicine immediately.
Critical missteps include self-adjusting doses without consultation and irregular post-dose eating patterns. Always access the paper patient leaflet inside packaging or MHRA online resources detailing side effects. Suspected adverse reactions require immediate Yellow Card scheme reporting via the MHRA website.
Practical Usage Guidance
Post-missed dose protocols: Take if remembered within 12 hours; skip if next dose approaches. Skipping occasionally proves safer than double-dosing. Driving restrictions apply only if experiencing dizziness – otherwise operate vehicles normally.
Common OTC precautions:
- Consult pharmacists before using ibuprofen (potential kidney strain)
- Avoid bulk-buying glucose tablets (accidental overconsumption risk)
- Declare all prescriptions when purchasing cold medicines (interaction screening)