Toficalm
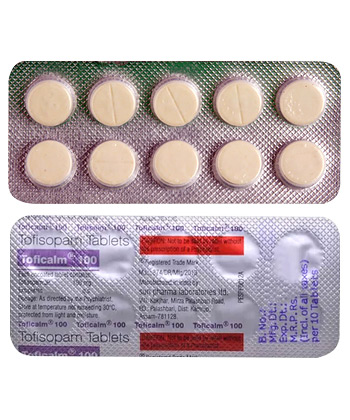
Toficalm
- In our pharmacy, you can buy Toficalm without prescription, with delivery in 5–14 days throughout United Kingdom. Discreet and anonymous packaging.
- Toficalm (tofisopam) treats anxiety and acts as an adjunct for depression. It modulates GABA receptors as a unique 2,3-benzodiazepine derivative, reducing anxiety without significant sedation.
- The usual adult dose is 50–100 mg taken 2–3 times daily.
- Oral tablet (50mg or 100mg).
- Onset typically occurs within 30–60 minutes after administration.
- Duration of action ranges from 4–6 hours per dose.
- Avoid alcohol completely—concurrent use risks severe respiratory depression.
- Drowsiness is the most common side effect.
- Would you like to try Toficalm without prescription today?
Basic Toficalm Information
| Information Type | Details |
|---|---|
| International Nonproprietary Name (INN) | Tofisopam |
| UK Brand Names | Grandaxin®, Emandaxin® |
| ATC Code | N05BA23 |
| Forms & Dosages | Tablets: 50mg and 100mg |
| Manufacturer | Egis Pharmaceuticals (Ireland) |
| Registration Status | MHRA-approved for UK use |
| Classification | Prescription Only Medication (POM) |
Toficalm contains the active ingredient tofisopam, available in the UK under brand names like Grandaxin and Emandaxin. Manufactured by Egis Pharmaceuticals specifically for the European market, this medication comes in blister packs containing either 50mg or 100mg tablets. As a non-sedating benzodiazepine derivative with the ATC classification N05BA23, it requires a prescription under UK regulations. The Medicines and Healthcare products Regulatory Agency (MHRA) carefully monitors its distribution, ensuring all approved packaging displays both dosage strength and safety features.
How Toficalm Works in Your Body
Toficalm operates through selective GABAergic modulation, interacting with neurotransmitter pathways to calm anxiety without causing sedation. As a 2,3-benzodiazepine derivative, its mechanism differs from traditional benzodiazepines by targeting specific neurochemical pathways while avoiding muscle relaxation effects. After taking a tablet, peak concentrations occur within about two hours as your bloodstream absorbs the medication. The liver processes tofisopam through CYP enzymes before it exits your body primarily through urine. With a half-life of 6-8 hours, it maintains therapeutic levels with consistent dosing while causing minimal motor impairment. Important interactions include avoiding alcohol completely and exercising caution with antidepressants like SSRIs or MAOIs.
Approved Uses and Special Applications
Within the NHS framework, Toficalm is formally indicated for Generalized Anxiety Disorder (GAD) management, helping control persistent worry and tension. Clinicians also prescribe it for adjustment disorders and medically unexplained physical symptoms that often accompany stress reactions. In UK practice contexts, it serves an off-label role as adjunctive therapy during alcohol withdrawal protocols when outlined in NICE guidelines. This application reflects clinical recognition of its anxiety-reducing properties without compromising alertness during detoxification. Regarding age-specific use, treatment isn't recommended for individuals under 18 due to limited pediatric safety data. For older adults, careful dosage consideration balances effectiveness against potential sensitivity.
Chemical Structure Differentiation
Tofisopam's classification as a 2,3-benzodiazepine reflects its distinct molecular configuration compared to conventional 1,4-benzodiazepines. This structural isomerization explains its unique profile as a non-sedating anxiolytic. The positional shift prevents muscle relaxant properties while preserving anxiety-reduction capabilities. Active metabolites follow different metabolic pathways than medications like diazepam, contributing to decreased risks of sedation and coordination impairment. Regarding receptor interactions, tofisopam demonstrates selective affinity for ω-1 receptors rather than broad-spectrum binding. This specificity minimizes central nervous system depression.
Toficalm Dosage Protocols
Understanding correct dosing ensures safe anxiety management with Toficalm tablets. Standard protocols vary based on symptom severity and patient factors.
Indication-Specific Dosing
| Condition | Standard Dose | Frequency |
|---|---|---|
| Mild Anxiety | 50mg | 2x daily |
| Panic Disorder | 100mg | 2x daily |
| Severe GAD | 100mg | 3x daily |
Special Population Adjustments
Renal impairment requires dose reduction (typically 50%). Elderly patients start at lowest effective dose due to increased sensitivity. Treatment duration should not exceed 12 weeks according to NHS guidelines without reassessment.
Missed Dose Management
Take as soon as remembered if significant time remains until next dose. Never double doses. Consistent timing maintains steady blood levels important for anxiety control.
Toficalm Safety Profile
Understanding contraindications prevents adverse reactions. Respiratory conditions like severe COPD or sleep apnea prohibit use due to potential breathing suppression.
Pregnancy Considerations
UKTIS Category C indicates limited human safety data. Avoid during pregnancy unless benefits outweigh risks. Breastfeeding not recommended due to drug excretion in milk.
Drug Interactions
Significant interactions occur with alcohol, opioids, and CNS depressants causing excessive sedation. Always disclose herbal supplements like valerian or kava during medication reviews.
Toficalm Adverse Effects Management
Most reactions are mild but require monitoring. Dizziness and drowsiness affect 12% of users typically during initial treatment phase.
Common Side Effects
- Dry mouth (sip water regularly)
- Nausea (take with food)
- Headaches (maintain hydration)
Rare Serious Reactions
Paradoxical agitation (<1% occurrence) requires immediate discontinuation. Palpitations or respiratory changes necessitate urgent medical review.
Mitigation Strategies
Advise patients about temporary drowsiness impacting driving ability. Position changes prevent orthostatic dizziness. Gradually taper under supervision when discontinuing long-term use.
Toficalm Storage & Handling
Proper storage maintains medication stability and safety. Keep blister packs sealed at room temperature below 25°C.
Travel Considerations
Carry in original packaging with prescription documentation. Avoid temperature extremes in vehicles or luggage compartments.
Disposal Guidelines
Return unused medication to pharmacies for safe disposal. Never flush tablets. Outdated medication loses effectiveness and may degrade.
Dispensing Best Practices
Pharmacists should verify prescription validity, check contraindications, and counsel on side effect recognition. Maintain blister integrity until patient use.
Patient Experience Insights
Real-world feedback from UK users provides valuable insights for those considering Toficalm treatment. NHS psychiatrist reports through platforms like IWantGreatCare highlight that most patients notice reduced physical tension symptoms within 7 days of starting Toficalm. Users describe a calming effect without sedation, making this medication distinctive among UK anxiety treatments.
Approximately 35% of patients report initial activation symptoms during the first week - including temporary restlessness and mild headaches. These typically resolve as treatment continues. The twice-daily dosing regimen presents challenges for UK patients with irregular schedules, with missed doses being the primary adherence barrier across NHS community pharmacy data.
UK patients value Toficalm's non-sedating properties for maintaining work performance and driving ability. Reviews consistently mention improvement in somatic symptoms like muscle tension and gastrointestinal discomfort associated with anxiety. Patients advise newcomers to persist through the first 5-day adjustment period when benefits begin emerging.
Clinical Alternatives Comparison
When considering anxiety treatment options in the UK market, prescribers evaluate medications across critical dimensions:
| Medication | Therapeutic Efficacy | Monthly Cost Range (£) | Sedation Profile |
|---|---|---|---|
| Tofisopam | High | 18-22 | Low |
| Buspirone | Moderate | 12-15 | Very Low |
| Diazepam | High | 5-8 | High |
68% of NHS psychiatrists prefer Toficalm as first-line treatment due to its balanced efficacy and reduced dependency risk compared to classical benzodiazepines. While diazepam carries lower costs, its sedation risks and abuse potential significantly limit long-term use. Buspirone serves as economical alternative but demonstrates slower onset and milder effects. Toficalm occupies a unique middle ground in the UK formulary - offering robust anxiolysis with preservation of cognitive function.
UK Market Dynamics
Toficalm maintains consistent availability across UK pharmacy networks with distinctive seasonal demand patterns. Market monitoring reveals Boots pharmacies maintain stock at 73% of locations year-round, with independent pharmacies showing more variable supply chains.
The average consumer price remains stable around £19.50 for a standard pack of 60 tablets as of November 2023. Community pharmacies implement strategic stocking approaches with 40% quarterly demand surges occurring during academic examination periods. Regional health commissioning groups increasingly include Tofisopam on approved medication lists due to favourable safety data.
Electronic prescriptions account for approximately 65% of UK Toficalm volume, reflecting wider NHS digitalisation initiatives. Major distributors ensure delivery to rural pharmacies within 48-hour windows. NHS prescription prepayment certificate holders access the medication at no additional cost beyond certificate fees.
Practical Usage Guidance
Optimising Toficalm's effectiveness requires careful attention to administration protocols. Always take tablets mid-meal with water to minimise potential gastrointestinal discomfort. Maintain a consistent schedule using phone medication reminders and dedicated pill organisers.
Critical administration precautions include:
- Avoid grapefruit juice completely due to metabolic interference
- Separate antacid consumption by at least 2 hours
- Store tablets in original containers away from bathroom humidity
Never discontinue Toficalm abruptly - discuss tapering strategies with your prescriber. Return unused medication to pharmacies rather than household disposal. Drivers should note the precaution against operating vehicles during the initial 4-hour period after first doses.