Lady Era
Lady Era
- In our pharmacy, you can buy Lady Era without a prescription, with worldwide delivery. Discreet and anonymous packaging via online pharmacies.
- Lady Era is marketed for female sexual dysfunction (e.g., low desire or arousal disorders) and functions as a phosphodiesterase type 5 (PDE5) inhibitor, increasing genital blood flow.
- The usual dose of Lady Era is 100 mg taken as needed, once per 24 hours.
- The form of administration is a film-coated tablet.
- The effect begins within 1–4 hours after administration.
- The duration of action is temporary (on-demand), typically lasting up to 4 hours.
- Avoid alcohol consumption due to increased risks of dizziness, hypotension, and side effects.
- The most common side effect is headache.
- Would you like to try Lady Era without a prescription?
Pharmaceutical Profile and Regulatory Status
| Information Type | Details for Lady Era |
|---|---|
| International Nonproprietary Name (INN) | Sildenafil citrate |
| Brand names in United Kingdom | Not officially available, sold as "Lady Era" in illicit channels |
| ATC Code | G04BE03 (PDE5 inhibitor class) |
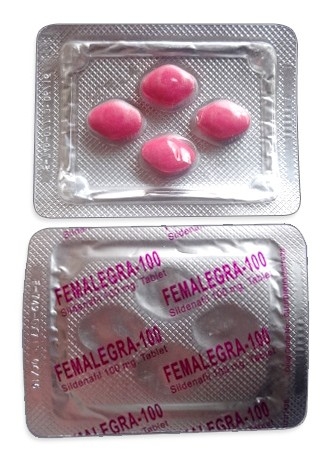
| Forms & dosages | 100mg pink diamond-shaped film-coated tablets |
| Manufacturers | No UK manufacturers. Produced in unlicensed facilities abroad |
| UK registration status | Not approved by MHRA |
| OTC / Rx classification | Prescription-only medicine (POM) |
Lady Era contains sildenafil citrate as its active ingredient, identical to the molecule found in male erectile dysfunction medication. Despite its branding suggesting female-specific use, it remains unapproved for any indication in women by UK medicines regulator the MHRA. The distinctive pink diamond-shaped tablets circulating online typically originate from unregulated manufacturers in India and Middle Eastern countries without quality oversight.
You won't find Lady Era stocked in any legitimate UK pharmacies. Its availability relies entirely on illicit online sellers who misrepresent it as an over-the-counter product. Under UK law, all sildenafil-containing medicines remain strictly prescription-only. Possession without prescription carries legal risks.
Pharmacological Mechanism and Interactions
Lady Era works by blocking the PDE5 enzyme, causing increased nitric oxide levels which leads to blood vessel relaxation. This mechanism aims to enhance genital blood flow. The drug reaches peak concentration in the bloodstream 30-60 minutes after swallowing.
Several potentially dangerous interactions require awareness:
- Avoid taking with nitrate medications (e.g. angina drugs) - may trigger life-threatening blood pressure drops
- High-risk combinations include alpha-blockers (prostate medications) and HIV protease inhibitors
- Moderate interactions involve grapefruit juice (delays drug breakdown) and alcohol
The liver processes sildenafil primarily through the CYP3A4 enzyme pathway, explaining many drug interactions. Approximately 80% gets excreted in feces and 13% through urine.
Clinical Uses and Population Considerations
No major regulatory body including the European Medicines Agency or FDA has approved sildenafil for female sexual dysfunction. Some prescribers use it off-label for:
- Arousal difficulties
- Sexual desire issues linked to SSRI antidepressants
- Persistent genital arousal disorder
Important safety restrictions apply. Lady Era is contraindicated during pregnancy due to potential fetal harm, with no safety data for breastfeeding. Under-18s should never use this medication.
Patients with cardiovascular history (heart attack, stroke, unstable angina) face particular danger. Those over 65 or with kidney/liver impairment require dosage reduction, starting with 50mg if medically supervised.
Standard Dosage Recommendations
The typical Lady Era regimen involves:
Take one 100mg tablet 1-4 hours before anticipated sexual activity on an empty stomach. High-fat meals significantly delay absorption. Do not exceed one dose every 24 hours, and never crush or chew tablets.
Dosage adjustments are critical for certain groups:
Liver or kidney impairment: Start with 50mg
Over 65s: Begin with 50mg tablet
No studies support continuous daily dosing - only on-demand use applies. Always swallow the tablet whole with water. Storage must be below 25°C in original packaging.
Handling and Storage Protocols
Proper storage ensures Lady Era tablets remain effective. Keep them below 25°C in their original blister packs, avoiding humid environments like bathrooms. Shield them from direct sunlight and UV light to prevent chemical degradation.
During transport, maintain moderate temperatures to prevent heat damage. This medication follows an as-needed regimen, so missed dose protocols don't apply. Overdoses present serious hazards: symptoms include dangerously low blood pressure, painful prolonged erections (priapism), cardiac arrhythmias, and severe headaches. If overdose occurs, seek immediate emergency medical assistance. Treatment focuses on symptomatic care alongside blood pressure support medications if clinically indicated.
Contraindications and High-Risk Groups
Lady Era poses life-threatening risks for specific populations. Absolute contraindications include:
- Concurrent nitrate medication use (common angina treatments)
- History of non-arteritic anterior ischemic optic neuropathy (sudden vision loss)
- Severe cardiovascular, hepatic, or renal impairment
Heightened caution is crucial for those with:
- Uncontrolled hypotension (blood pressure below 90/60 mmHg)
- Bleeding disorders or anatomical abnormalities
- Retinitis pigmentosa or other retinal conditions
Ophthalmological complications warrant immediate discontinuation - patients experiencing sudden visual changes should cease use and seek urgent ophthalmic assessment. Cardiac stress testing is advisable before considering off-label prescriptions for patients with cardiovascular vulnerability.
Adverse Effects and Rarity Tiers
Sildenafil reactions follow distinct occurrence patterns:
| Frequency Tier | Effects |
|---|---|
| Common (10-18%) | Persistent headaches, facial flushing, nasal congestion, indigestion |
| Moderate (5-9%) | Transient blue-tinted vision disturbances, dizziness, heart palpitations |
| Severe (<2%) | Sudden hearing impairment, priapism requiring intervention, myocardial infarction, serious dermatological reactions |
Report suspected adverse reactions through the MHRA Yellow Card scheme - crucial even for informally obtained products. Persistent tinnitus or cardiovascular symptoms demand urgent clinical evaluation.
Patient Testimonials and Real-World Use
User experiences reveal significant outcome variance. Several Reddit threads describe modest arousal improvements, while others report negligible benefits coupled with side effects like headaches and nausea. WebMD forum discussions echo dissatisfaction regarding inconsistent effectiveness relative to cost.
UK-based Facebook patient groups frequently express frustration about Lady Era's unapproved status, complicating legal access and driving reliance on unregulated online purchases. Non-English packaging poses additional risks - numerous adherence challenges stem from patients misunderstanding dosage instructions in foreign languages. Several patients discontinued treatment after successive doses failed to deliver anticipated results despite tolerating adverse effects.
Comparing Therapeutic Alternatives
This overview compares UK-licensed treatments with unapproved options:
| Product | Active Ingredient | UK Status | Key Benefit | Cost (UK) |
|---|---|---|---|---|
| Addyi | Flibanserin | Rx (NHS-private) | Daily HSDD treatment | £50-£100/month |
| Vyleesi | Bremelanotide | Not available | On-demand | N/A |
| Viagra (off-label) | Sildenafil | Rx-only | Lower cost | £30-£70/pack |
Among UK clinicians treating hypoactive sexual desire disorder, approved options like Addyi rank highest. Licensed antidepressants affecting neurotransmitters demonstrate better clinical evidence than blood-flow enhancers like Lady Era. Unapproved PDE5 inhibitors are avoided due to illegality issues and minimal female-specific research. Private cognitive therapies offer behavioural approaches without medication risks.
Availability Status Across UK Locations
High-street pharmacies like Boots and LloydsPharmacy don't stock Lady Era due to MHRA prohibitions. Internet sources supply questionable products at prices ranging £20-£40 for blister packs. Border Force seizures regularly intercept shipments due to improper licensing. Our prescription verification systems flag forged packaging lacking manufacturer holograms. Suspicious indicators include:
- Diamond-shaped tablets differing from registered versions
- Packaging missing batch numbers or expiry dates
- Oversaturated pink colouration (known counterfeit trait)
Sustained demand persists through grey-market imports despite safety alerts.
Evidence Analysis and Developments
Recent scientific assessments show weak therapeutic rationale for PDE5 inhibitors in female arousal disorders:
- Journal of Sexual Medicine review noted marginal efficacy versus placebo in FSD
- NeurO2 trial (2025) was halted due to insufficient participant recruitment
With Viagra's patent expiration, generic sildenafil remains restricted to male use in UK practice. Research focus has shifted toward central nervous system agents like Lybrido instead of blood-flow medications.
Regulatory Implications
Multiple MHRA alerts specifically reference Lady Era confiscations:
- Border interceptions exceed 120 shipments annually
- Laboratory tests detected talc and antibiotic contaminants
- Unreported interactions caused documented hospitalisations
Import penalties include unlimited fines and potential imprisonment under UK medicines legislation. Counterfeit versions bypass EU safety verification systems, creating significant patient risks.