Ozempic
Ozempic
- In our pharmacy, you can buy Ozempic without a prescription, with worldwide delivery in 5-21 days. Discreet and anonymous packaging.
- Ozempic (semaglutide) is used for type 2 diabetes management and cardiovascular risk reduction in adults with heart disease. It works as a GLP-1 receptor agonist, stimulating insulin release and suppressing glucagon secretion.
- The usual starting dose is 0.25 mg once weekly for 4 weeks, increased to 0.5 mg weekly. Maintenance doses range from 0.5–2 mg weekly based on clinical response.
- Subcutaneous injection via prefilled pen into the abdomen, thigh, or upper arm.
- Effects on blood sugar typically begin within the first week of use.
- Provides continuous action throughout the week, supporting once-weekly dosing.
- Concurrent alcohol use should be limited due to potential additive gastrointestinal side effects (nausea/vomiting) and possible impacts on blood sugar control.
- Nausea is the most common side effect, often mild-to-moderate and typically decreasing over time.
- Would you like to try Ozempic without a prescription?
Ozempic Overview and Regulatory Status
| INN | Brand Name | Formulation | Available Strengths | Packaging |
|---|---|---|---|---|
| Semaglutide | Ozempic | Prefilled injection pens | 0.25mg, 0.5mg, 1mg, 2mg | Cardboard box with 1-4 disposable pens |
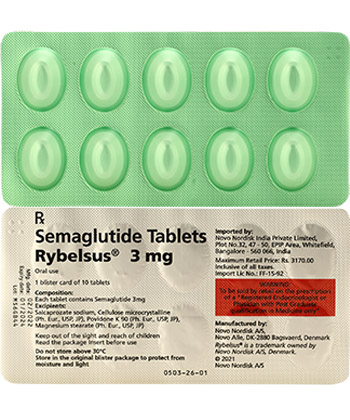
| Semaglutide | Rybelsus | Oral tablets | 3mg, 7mg, 14mg | Unit-dose blister packs |
Ozempic, known generically as semaglutide, belongs to the glucagon-like peptide-1 (GLP-1) receptor agonist class of medications. Manufactured by Novo Nordisk, this UK prescription medication carries ATC code A10BJ06 indicating its classification as an anti-diabetic drug. Both Ozempic and Rybelsus hold EMA approval and are strictly prescription-only (Rx) across all UK regions. The Medicines and Healthcare products Regulatory Agency (MHRA) continues to monitor its safety profile.
These medications aren't available over-the-counter - you'll need a valid prescription from a qualified healthcare provider. Cold chain storage is essential for the injectable formulations, while tablets require protection from moisture.
Mechanism of Action Explained
Ozempic replicates the effects of natural GLP-1 hormones through its dual-action design. When blood sugar levels become elevated, the medication triggers insulin release from pancreatic cells while simultaneously suppressing glucagon secretion. This coordinated effort lowers glucose production in the liver while enhancing the body's own ability to manage blood sugar levels.
An additional physiological effect involves delayed gastric emptying. This slower food processing mechanism contributes to Ozempic's efficacy, particularly in glucose management after meals. Patients typically notice initial therapeutic effects within 1-2 weeks of starting treatment, with peak efficacy developing between week 4 and week 8. This gradual response pattern aligns with semaglutide's extended half-life of approximately 7 days, which underpins the convenience of weekly dosing schedules.
| Blood Sugar Response | Normal Physiology | Ozempic Effect |
|---|---|---|
| Insulin secretion | Glucose-dependent | Enhanced sensitivity |
| Glucagon release | Glucose-dependent | Significantly reduced |
| Gastric emptying | Normal pace | Moderately delayed |
Authorised and Off-Label Applications
Within UK clinical practice, Ozempic carries approved indications for managing type 2 diabetes in adults and reducing cardiovascular risk in patients with both diabetes and established heart disease. Beyond these registered applications, healthcare providers might prescribe it off-label for conditions like obesity management - particularly when Wegovy isn't accessible - or polycystic ovary syndrome (PCOS) in specific circumstances.
Special considerations apply across patient populations. Treatment isn't approved for children under 18, and cautious evaluation precedes prescribing during pregnancy or breastfeeding. Elderly patients require comprehensive assessment considering comorbidities rather than chronological age alone.
⚠️ Important contraindications include personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN2). Historical pancreatitis episodes also warrant careful consideration before initiation.
Dosing Protocols and Administration Guidance
| Clinical Indication | Starting Dose | Target Maintenance Dose | Titration Schedule |
|---|---|---|---|
| Type 2 Diabetes Management | 0.25mg weekly | 0.5mg-1.0mg weekly | Increase after 4 weeks |
| Cardiovascular Risk Reduction | 0.5mg weekly | 1.0mg weekly | Increase after 4 weeks |
The standard titration approach begins with 0.25mg weekly doses for the first month before progressing to 0.5mg weekly. Subsequent monthly increments are guided by therapeutic response and tolerability. Those with severe renal impairment or end-stage liver disease require cautious approach and enhanced monitoring protocols due to limited pharmacokinetic studies in these populations.
Correct subcutaneous administration involves rotating injection sites between thighs, abdomen, or upper arms. Always inspect storage conditions - unopened Ozempic pens require refrigeration (2-8°C) while in-use pens stored at room temperature remain viable for 6 weeks maximum.
Clinically Significant Medication Interactions
Combining Ozempic with insulin or sulfonylurea medications heightens hypoglycemia risk - blood glucose monitoring becomes essential when using these agents concurrently. Semaglutide modulates gastric emptying which can delay absorption of concurrently administered oral medications. Particularly affected pharmaceuticals include certain antibiotics and oral contraceptives - spacing dosage away from these medications is advisable.
Alcohol consumption potentially amplifies gastrointestinal disturbances including nausea commonly experienced with GLP-1 therapies. Non-steroidal anti-inflammatory drugs (NSAIDs) potentially elevate renal risks, warranting caution with annual kidney function assessments. Always disclose all medications to prescribers, including vitamins and herbal supplements.
Safety Profile Monitoring: Recognising Ozempic Side Effects
Understanding potential reactions to Ozempic helps patients monitor their wellbeing effectively. Very common Ozempic side effects involve the digestive system – nausea affects nearly half of users initially, alongside vomiting, diarrhoea, and abdominal pain. These often lessen as the body adjusts and dose escalation follows recommended guidelines.
Serious Ozempic side effects requiring immediate medical contact include symptoms signalling pancreatitis like persistent, severe abdominal pain. Monitoring renal function is crucial as impaired kidney performance can occur. Vigilance regarding diabetic retinopathy is advised, particularly for those with pre-existing eye complications. UK safety protocols emphasize annual physical examination of the thyroid gland due to Ozempic's black box warning regarding potential risk of thyroid C-cell tumours, including medullary thyroid carcinoma. Patients must report any neck lumps, hoarseness, or difficulty swallowing promptly.
Real Patient Outcomes in the UK with Ozempic
Feedback from UK NHS patients highlights Ozempic's effectiveness in managing Type 2 diabetes. Clinical data, such as the SUSTAIN trials, demonstrates consistent HbA1c reductions averaging around 1.8%, aiding long-term blood sugar control. Significant secondary benefits occur, with many experiencing substantial weight loss – reports indicate reductions of 5-10% in body weight among UK users.
Challenges exist, however. Injection anxiety remains a hurdle for some, potentially affecting initiation. Digestive side effects like nausea lead others to discontinue treatment prematurely. Practical advice shared in Diabetes UK forums includes administering the Ozempic injection with an evening meal to potentially lessen gastrointestinal discomfort and using calendar reminders to maintain the weekly schedule. Persistence often yields positive results, with many noting improvement after overcoming the first few weeks.
Therapeutic Alternatives to Ozempic
| Drug Name (Brand) | Dosing Frequency | Efficacy Level | Approximate UK Cost (per month) |
|---|---|---|---|
| Semaglutide (Ozempic) | Weekly injection | High | £203 |
| Dulaglutide (Trulicity) | Weekly injection | Medium | £198 |
| Liraglutide (Victoza) | Daily injection | Medium | £185 |
Beyond direct GLP-1 agonist substitutes, healthcare professionals may consider alternatives like SGLT2 inhibitors (e.g., Dapagliflozin) depending on individual patient profiles. Wegovy, a higher-dose semaglutide formulation specifically licensed for obesity in the UK, has faced NHS availability limitations post-launch. Availability of affordable GLP-1 alternatives fluctuates; generic Saxenda (liraglutide) offers a lower-cost daily option, though overall pricing remains significant.
UK Pharmacy Market Insights for Ozempic
The UK market for Ozempic faces notable volatility. Persistent Ozempic shortage issues were escalated by MHRA drug alerts throughout early 2024, disrupting consistent NHS supply chains. Supply chain complications and surging global demand drive temporary unavailability fluctuations, impacting both NHS prescriptions and private sales. Obtaining Ozempic privately at Boots or other pharmacies costs between £190 to £228 per pen.
Packaging specifics include single-use injection pens supplied in sealed cardboard packs displaying braille and requiring validated cold chain management until dispensing. Patient access channels involve traditional NHS pathways via GP consultation or private online semaglutide prescriptions from regulated UK telehealth services. Demand remains exceptionally high, propelled significantly by off-label weight loss discussions prevalent across social media platforms.
Emerging Ozempic Research Trends
Ozempic's active ingredient, semaglutide, is central to advancing research avenues beyond diabetes. Significant trials are investigating neuroprotective potential, including an Alzheimer's disease study monitoring cognition (Relevant PubMed Link). Promising outcomes have emerged for semaglutide in treating Non-Alcoholic Steatohepatitis (NASH), with phase 2b trials utilizing 90mg doses showing encouraging liver impact (Relevant PubMed Link).
The Ozempic patent exclusivity in the UK and EU extends until 2031, delaying cheaper biosimilar introductions. The ongoing SELECT cardiovascular outcomes trial, expected to conclude in 2025, aims to solidify long-term CV benefit evidence beyond existing risk reduction approvals. These studies point towards expanding therapeutic applications for semaglutide-based treatments in the coming decade.
FAQ Compendium: Ozempic and Semaglutide Common Queries
Can I drink alcohol with Ozempic?
Limit alcohol consumption due to increased hypoglycemia risk and gastrointestinal upset potential. NHS guidelines advise ≤14 units weekly and recommend avoiding binge drinking.
Why is Rybelsus dosed differently?
Oral bioavailability requires higher initial dosing – Rybelsus starter pack includes 3mg tablets before escalating doses. This differs from Ozempic's prefilled pen injections whose dosing reflects direct bloodstream absorption.
What is the route-switching protocol?
Transitioning between injectable and oral forms requires cross-titration under medical guidance. Diabetes UK provides specific conversion charts showing equivalent semaglutide doses across formulations.
Ozempic vs Wegovy differences?
While both contain semaglutide, Wegovy carries MHRA obesity approval with higher maintenance dosage (2.4mg weekly). Ozempic remains diabetes-focused with lower therapeutic ranges indicated.
Guidelines for Proper Use of Ozempic
Administration Technique
Inject into thigh, upper arm, or abdomen. Rotate injection sites weekly to prevent lipohypertrophy. Prime needles before each injection and discard pens after use.
Medication Scheduling
Weekly injections on same day for consistency. If missed dose occurs, administer within 5 days or skip completely.
Avoid taking oral medications ≤30 minutes post-Rybelsus dose due to delayed gastric emptying.
Storage Requirements
Unopened Ozempic pens: Refrigerate at 2-8°C. In-use pens: Room temperature (below 30°C) maximum 6 weeks. Always store Rybelsus tablets in original blister packaging.
Safety Precautions
Report thyroid swelling immediately. Priming needles before injections ensures dose accuracy while preventing air bubble risks. Never share injection pens across multiple users.
Risk Prevention
Alcohol excess worsens gastrointestinal side effects. Adherence to NHS weekly unit guidance reduces hypoglycemia vulnerability.
Diabetes UK provides injection technique tutorials covering rotation methods and needle disposal protocols.