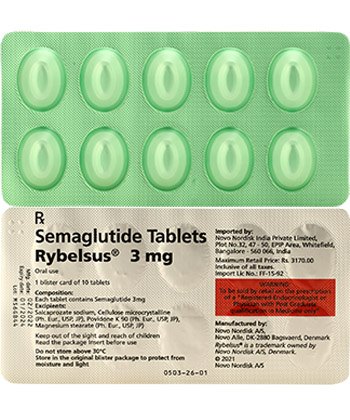
Rybelsus
Rybelsus
- In our pharmacy, you can buy Rybelsus without a prescription, with worldwide delivery in 5-21 days. Discreet and anonymous packaging.
- Rybelsus (semaglutide) is used for type 2 diabetes management and cardiovascular risk reduction in adults with heart disease. It works as a GLP-1 receptor agonist, stimulating insulin release and suppressing glucagon secretion.
- The usual starting dose is 0.25 mg once weekly for 4 weeks, increased to 0.5 mg weekly. Maintenance doses range from 0.5–2 mg weekly based on clinical response.
- Subcutaneous injection via prefilled pen into the abdomen, thigh, or upper arm.
- Effects on blood sugar typically begin within the first week of use.
- Provides continuous action throughout the week, supporting once-weekly dosing.
- Concurrent alcohol use should be limited due to potential additive gastrointestinal side effects (nausea/vomiting) and possible impacts on blood sugar control.
- Nausea is the most common side effect, often mild-to-moderate and typically decreasing over time.
- Would you like to try Rybelsus without a prescription?
Trackable delivery
9-21 days
Payment method
Visa, Mastercard, Discovery, Bitcoin, Ethereum
Free delivery (by Standard Airmail) on orders over £150
Related Products:
Rybelsus Overview and Regulatory Status
| INN | Brand Name | Formulation | Available Strengths | Packaging |
|---|---|---|---|---|
| Semaglutide | Ozempic | Prefilled injection pens | 0.25mg, 0.5mg, 1mg, 2mg | Cardboard box with 1-4 disposable pens |
| Semaglutide | Rybelsus | Oral tablets | 3mg, 7mg, 14mg | Unit-dose blister packs |
Mechanism of Action Explained
Rybelsus replicates the effects of natural GLP-1 hormones through its dual-action design. When blood sugar levels become elevated, the medication triggers insulin release from pancreatic cells while simultaneously suppressing glucagon secretion. This coordinated effort lowers glucose production in the liver while enhancing the body's own ability to manage blood sugar levels. An additional physiological effect involves delayed gastric emptying. This slower food processing mechanism contributes to Rybelsus's efficacy, particularly in glucose management after meals. Patients typically notice initial therapeutic effects within 1-2 weeks of starting treatment, with peak efficacy developing between week 4 and week 8. This gradual response pattern aligns with semaglutide's extended half-life of approximately 7 days, which underpins the convenience of weekly dosing schedules.| Blood Sugar Response | Normal Physiology | Rybelsus Effect |
|---|---|---|
| Insulin secretion | Glucose-dependent | Enhanced sensitivity |
| Glucagon release | Glucose-dependent | Significantly reduced |
| Gastric emptying | Normal pace | Moderately delayed |
Authorised and Off-Label Applications
Within UK clinical practice, Rybelsus carries approved indications for managing type 2 diabetes in adults and reducing cardiovascular risk in patients with both diabetes and established heart disease. Beyond these registered applications, healthcare providers might prescribe it off-label for conditions like obesity management - particularly when Wegovy isn't accessible - or polycystic ovary syndrome (PCOS) in specific circumstances. Special considerations apply across patient populations. Treatment isn't approved for children under 18, and cautious evaluation precedes prescribing during pregnancy or breastfeeding. Elderly patients require comprehensive assessment considering comorbidities rather than chronological age alone. Important contraindications include personal or family history of medullary thyroid carcinoma (MTC) or multiple endocrine neoplasia syndrome type 2 (MEN2). Historical pancreatitis episodes also warrant careful consideration before initiation.Dosing Protocols and Administration Guidance
| Clinical Indication | Starting Dose | Target Maintenance Dose | Titration Schedule |
|---|---|---|---|
| Type 2 Diabetes Management | 0.25mg weekly | 0.5mg-1.0mg weekly | Increase after 4 weeks |
| Cardiovascular Risk Reduction | 0.5mg weekly | 1.0mg weekly | Increase after 4 weeks |
Clinically Significant Medication Interactions
Combining Rybelsus with insulin or sulfonylurea medications heightens hypoglycemia risk - blood glucose monitoring becomes essential when using these agents concurrently. Semaglutide modulates gastric emptying which can delay absorption of concurrently administered oral medications. Particularly affected pharmaceuticals include certain antibiotics and oral contraceptives - spacing dosage away from these medications is advisable. Alcohol consumption potentially amplifies gastrointestinal disturbances including nausea commonly experienced with GLP-1 therapies. Non-steroidal anti-inflammatory drugs (NSAIDs) potentially elevate renal risks, warranting caution with annual kidney function assessments. Always disclose all medications to prescribers, including vitamins and herbal supplements.Safety Profile Monitoring: Recognising Rybelsus Side Effects
Understanding potential reactions to Rybelsus helps patients monitor their wellbeing effectively. Very common Rybelsus side effects involve the digestive system – nausea affects nearly half of users initially, alongside vomiting, diarrhoea, and abdominal pain. These often lessen as the body adjusts and dose escalation follows recommended guidelines. Serious Rybelsus side effects requiring immediate medical contact include symptoms signalling pancreatitis like persistent, severe abdominal pain. Monitoring renal function is crucial as impaired kidney performance can occur. Vigilance regarding diabetic retinopathy is advised, particularly for those with pre-existing eye complications. UK safety protocols emphasize annual physical examination of the thyroid gland due to Rybelsus's black box warning regarding potential risk of thyroid C-cell tumours, including medullary thyroid carcinoma. Patients must report any neck lumps, hoarseness, or difficulty swallowing promptly.Real Patient Outcomes in the UK with Rybelsus
Feedback from UK NHS patients highlights Rybelsus's effectiveness in managing Type 2 diabetes. Clinical data, such as the SUSTAIN trials, demonstrates consistent HbA1c reductions averaging around 1.8%, aiding long-term blood sugar control. Significant secondary benefits occur, with many experiencing substantial weight loss – reports indicate reductions of 5-10% in body weight among UK users. Challenges exist, however. Injection anxiety remains a hurdle for some, potentially affecting initiation. Digestive side effects like nausea lead others to discontinue treatment prematurely. Practical advice shared in Diabetes UK forums includes administering the Rybelsus injection with an evening meal to potentially lessen gastrointestinal discomfort and using calendar reminders to maintain the weekly schedule. Persistence often yields positive results, with many noting improvement after overcoming the first few weeks.Therapeutic Alternatives to Rybelsus
| Drug Name (Brand) | Dosing Frequency | Efficacy Level | Approximate UK Cost (per month) |
|---|---|---|---|
| Semaglutide (Rybelsus) | Weekly injection | High | £203 |
| Dulaglutide (Trulicity) | Weekly injection | Medium | £198 |
| Liraglutide (Victoza) | Daily injection | Medium | £185 |